The interdisciplinary work in the Gustavsson Lab is focused on the development and application of 3D single-molecule tracking and super-resolution imaging throughout mammalian cells. Below we provide an overview of some of our methods and applications. For more detailed information, please see the Publications page for the references listed below.
Technical development
We push the limits of cellular imaging by developing and combining multiple different techniques.
Single particle tracking and single-molecule super-resolution imaging
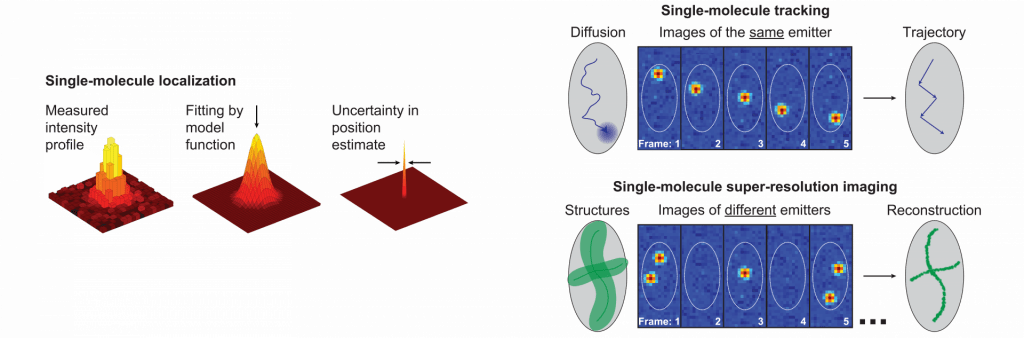
Single particle tracking (SPT) and single-molecule super-resolution (SR) imaging are methods that apply single-emitter localization to answer key questions in both biology and materials science. This approach circumvents the diffraction limit that otherwise limits image resolution to 200−300 nm in conventional fluorescence microscopy, and yields information on spatial scales down to tens of nanometers and on dynamics in the millisecond range in the case of SPT. We use point spread function engineering (see below) to achieve SPT and SR imaging in 3D throughout whole cells.
In a SPT experiment, the spatial trajectory of a given molecule is determined by repeatedly detecting and localizing it at many sequential time points. Analysis of the resulting single-molecule trajectories provides information on the mode of motion, which may be diffusive, motor-directed, confined, or a mixture of these modes, as well as information on potential molecular interactions.
In SR imaging, the positions of many different molecules are determined at different times to resolve a static structure. Here, the experimenter must actively use a photophysical, photochemical, or other mechanism which forces most of the emitters to be off while only a very small, non-overlapping subset is on in each camera frame. The positions recorded from many different camera frames randomly sample the labeled structure, and a point-by-point reconstruction can then be assembled by combining the localized positions of all detected molecules in a computational post-processing step.
8. Shechtman et al., Biomed. Opt. Express 8 5735-5748 (2017) [link]
11. Gustavsson et al., Opt. Express 26 13122-13147 (2018) [link]
12. Gustavsson et al., Nat. Commun. 9 1-8 (2018) [link]
Figure adapted with permission from Diezmann et al. Chem. Rev.117, 7244–7275 (2017)
Copyright 2017 American Chemical Society
Light sheet illumination
A powerful approach to improve imaging in thick cells is to use light sheet illumination, where the sample is excited by a thin sheet of light orthogonal to the detection axis. Light sheet illumination allows for selective irradiation of the image plane, and its inherent optical sectioning capability allows for imaging of biological samples with reduced fluorescence background, photobleaching, and photodamage.
We have developed Tilted light sheet microscopy with 3D point spread functions (TILT3D), which combines a tilted light sheet illumination strategy with long axial range point spread functions (see below) for low-background, 3D super-localization of single molecules as well as 3D super-resolution imaging in thick cells. We have used TILT3D for SR imaging of various cellular structures, including the nuclear lamina shown below.
11. Gustavsson et al., Opt. Express 26 13122-13147 (2018) [link]
12. Gustavsson et al., Nat. Commun. 9 1-8 (2018) [link]
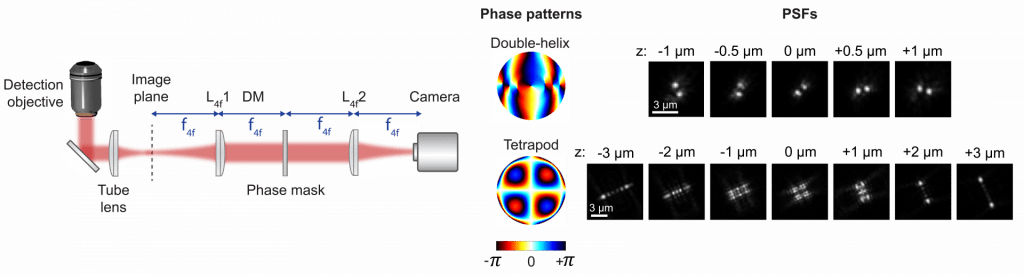
Point spread function engineering
We use engineered point spread functions (PSFs) to localize molecules or particles in all three dimensions. The strategy is to modify the shape of the PSF to encode information about the axial (z) position of each fluorescent molecule directly in its image, which is accomplished by modifying the phase pattern of the emitted light in the Fourier plane of the microscope. PSF engineering only requires the addition of a small number of optical elements to the collection path of a standard microscope, making the method relatively simple to implement while exhibiting high precision for 3D single-molecule localization. This method has been used to create several different PSFs, including the Double-helix PSF with axial ranges of ~2–3 μm, and the recently developed Tetrapod PSFs, which have a tunable range of up to 20 μm.
8. Shechtman et al., Biomed. Opt. Express 8 5735-5748 (2017) [link]
11. Gustavsson et al., Opt. Express 26 13122-13147 (2018) [link]
12. Gustavsson et al., Nat. Commun. 9 1-8 (2018) [link]
13. Bayas et al., Prot. Exchange, (2019) [link], [software download]
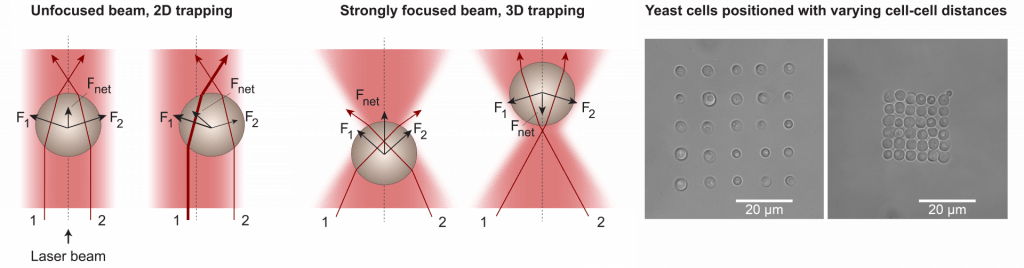
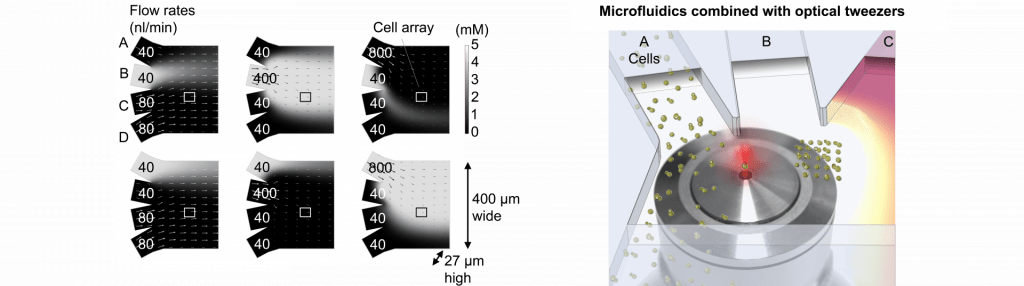
Optical tweezers
Optical tweezers are tools that use a strongly focused laser beam to directly trap, move and position micron-sized objects. We have paired optical tweezers with microfluidics (see below) for studies of rhythms in and communication between individual yeast cells. Optical tweezers enable precise positioning of cells with well-defined cell-cell distances. They can also be used to measure forces for rheological studies.
2. Gustavsson et al., FEBS J. 279 2837-2847 (2012) [link]
4. Gustavsson et al., FEBS Lett. 588 3-7 (2014) [link]
5. Gustavsson et al., FEBS J. 281 2784-2793 (2014) [link]
6. Gustavsson et al., Sci. Rep. 5 1-7 (2015) [link]
10. Gustavsson et al., Curr. Prot. Cell Biol. e70, 1-26 (2018) [link]
14. van Niekerk et al., Biochem. J. 476 353-363 (2019) [link]
Microfluidics
In microfluidics, we deal with fluid flows in channels where at least one dimension is in the micrometer range. Flows in such channels can become completely laminar, without any turbulence, and the only way for solutions from different channels to mix is by diffusion. This useful property allows us to perform fast, reversible changes of the extracellular environment of cells inside of the microfluidic chamber by varying the flow rates in different inlet channels. The cell responses to various treatments, including novel drugs, can then be studied in real time under the microscope.
2. Gustavsson et al., FEBS J. 279 2837-2847 (2012) [link]
4. Gustavsson et al., FEBS Lett. 588 3-7 (2014) [link]
5. Gustavsson et al., FEBS J. 281 2784-2793 (2014) [link]
6. Gustavsson et al., Sci. Rep. 5 1-7 (2015) [link]
10. Gustavsson et al., Curr. Prot. Cell Biol. e70, 1-26 (2018) [link]
14. van Niekerk et al., Biochem. J. 476 353-363 (2019) [link]
Applications
We apply the techniques above to answer biophysical and biomedical questions related to aging, cancer, and other diseases. Below we describe a few examples of our applied studies.
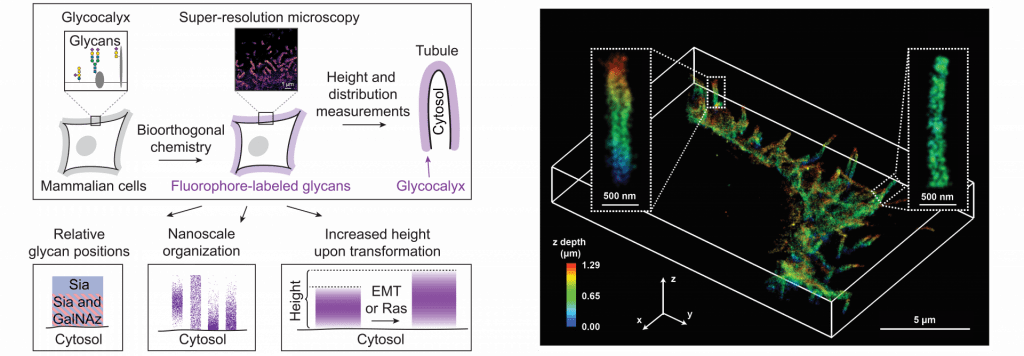
Determining the role of the glycocalyx nanoscale structure and organization in cancer progression
The mammalian glycocalyx is a heavily glycosylated extramembrane compartment found on nearly every cell. It is the first component of a cell to interact with the environment and therefore plays an important role in cell-cell and cell-matrix interactions critical to embryonic development, immune cell trafficking, cancer progression, and many other normal and pathological processes. Despite its relevance in both health and disease, studies of the glycocalyx remain hampered by a paucity of methods to spatially classify its components.
We combine metabolic labeling, bioorthogonal chemistry, and single-molecule super-resolution microscopy to image two constituents of the glycocalyx, N-acetylgalactosamine (GalNAc) and sialic acid, with 10–20 nm precision in 2D and 3D. This approach enables two measurements: the distribution of individual sugars distal from the membrane and the glycocalyx height. These measurements show that the glycocalyx exhibits nanoscale organization on both cell lines and primary human tumor cells. Additionally, we observe enhanced glycocalyx height in response to epithelial-to-mesenchymal transition (EMT) and to oncogenic KRAS activation. In the latter case, we trace increased height to an effector gene, GALNT7. These data highlight the power of advanced imaging methods to provide molecular and functional insights into glycocalyx biology.
15. Möckl et al., Dev. Cell 50 1-16 (2019) [link]
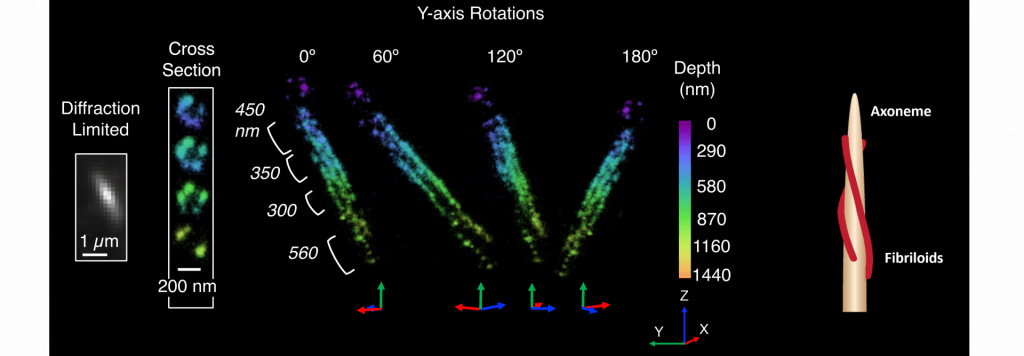
Discovering a novel structure in the primary cilium
The primary cilium is a signaling organelle that projects from the surfaces of specific cell types, including many human tissues. Primary cilia contain a sub-compartment called the inversin compartment, to which four proteins are known to localize: INVS, ANKS6, NEK8, and NPHP3. The function of the inversin compartment is unknown, but it appears to be critical for normal development, including left–right asymmetry and renal tissue homeostasis.
Here we combine super-resolution imaging of human RPE1 cells with a genetic dissection of the protein–protein binding relationships that organize compartment assembly to develop a new structural model. We observe that INVS is the core structural determinant of a compartment composed of novel fibril-like substructures, “fibriloids”. We find that NEK8 and ANKS6 depend on INVS for localization to these fibrillar assemblies and that ANKS6-NEK8 density within the compartment is regulated by NEK8. Together, NEK8 and ANKS6 are required downstream of INVS to localize and concentrate NPHP3 within the compartment. In the absence of these upstream components, NPHP3 is redistributed within cilia. These results provide a more detailed structure for the inversin compartment and introduce a new example of a membraneless compartment organized by protein–protein interactions.
16. Bennett, Gustavsson, et al., Mol. Biol. Cell 31 619-639 (2019) [link]